Why can't I change the subscripts in a molecule when I balance a chemical reaction?
1 Answer
Dec 5, 2016
Because you change the integrity of the compound you're looking at.
For example:
#6"HCl" + 2"Al"(s) -> 2"AlCl"_3 + 3"H"_2(g)#
If you suddenly write
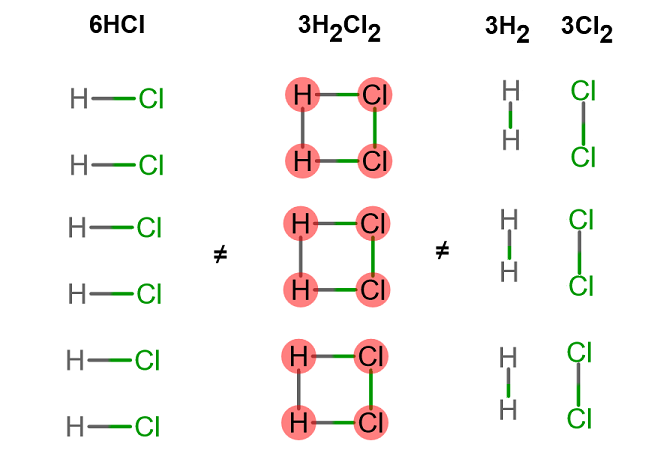
The new chemical formula of
Likewise,

