Question #23e3b
1 Answer
Jan 21, 2017
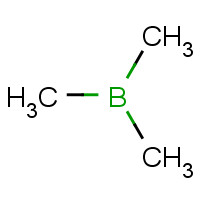
Trimethylborane.
Explanation:
So, you must find the name of
#(color(blue)("CH"_3))_color(red)(3)"B"#
Notice that the compound given to you contains
You can think of these methyl groups as replacing the hydrogen atoms in borane,
Instead of a central boron atom bonded to three hydrogen atoms, you have it bonded to three carbon atoms.
The show that you're dealing with
You will have
#(color(blue)("CH"_3))_color(red)(3)"B" -> color(red)("tri")color(blue)("methyl")"borane"#