What is the highest number of covalent bonds observed?
1 Answer
A common maximum number of covalent bonds is
- its
#2p_z# orbital to make a sigma (#sigma# ) bond - its
#2p_x# orbital to make one of the pi (#pi# ) bonds - its
#2p_y# orbital to make one of the pi (#pi# ) bonds
Thus, three bonds are made in total: one
#:"N"-="N":#
However, in the

As usual, the first three bonds are one
- one
#d_(z^2)# -#d_(z^2)# #bbsigma# overlap - one
#d_(xz)# -#d_(xz)# #bbpi# overlap - one
#d_(yz)# -#d_(yz)# #bbpi# overlap - either a
#d_(xy)# -#d_(xy)# or a#d_(x^2-y^2)# -#d_(x^2-y^2)# #bbdelta# overlap
Quintuple bonds are rare but one of these can be seen below.
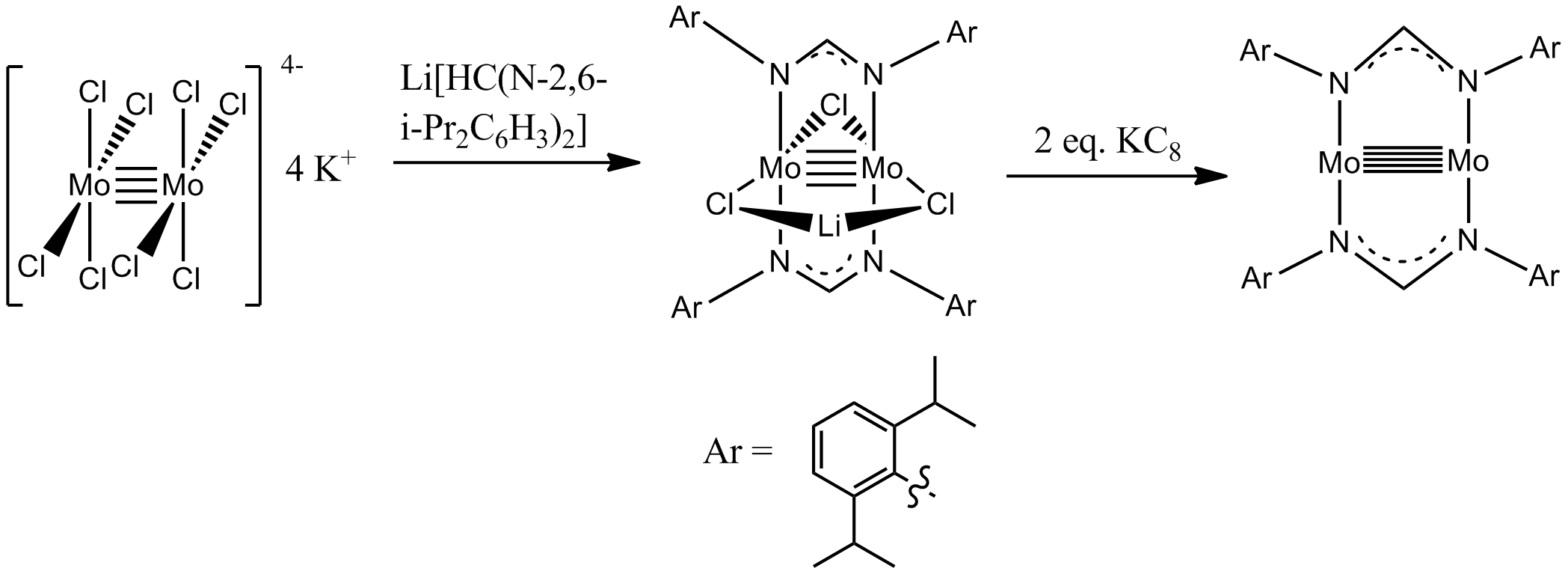
Bond length:
#~~# #"202 pm"# .(Note:
#"Ar"# probably just means aromatic ring, not argon.)
It would have one

