Question #8462f
1 Answer
Here's what I get.
Explanation:
Sodium is the only Group 1 element that gives a yellow flame colour.
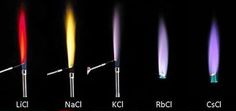
(Adapted from www.pinterest.com)
A sodium atom in an unexcited state has the electron configuration
The bright orange-yellow flame colour results when electrons fall back from the
The most likely candidate for the green gas is chlorine.

Sodium reacts vigorously with chlorine to form the white solid, sodium chloride.

The melting point of the ionic solid is 801 °C.
The equation for the reaction is
A sodium ion,
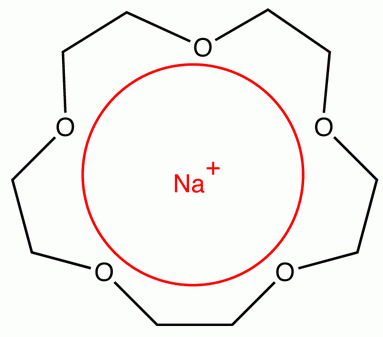
(Adapted from OChemPal)
The crown ether is soluble in organic solvents, so the complex is often used to make sodium salts soluble in organic solvents.

