Electron Configuration
Key Questions
-
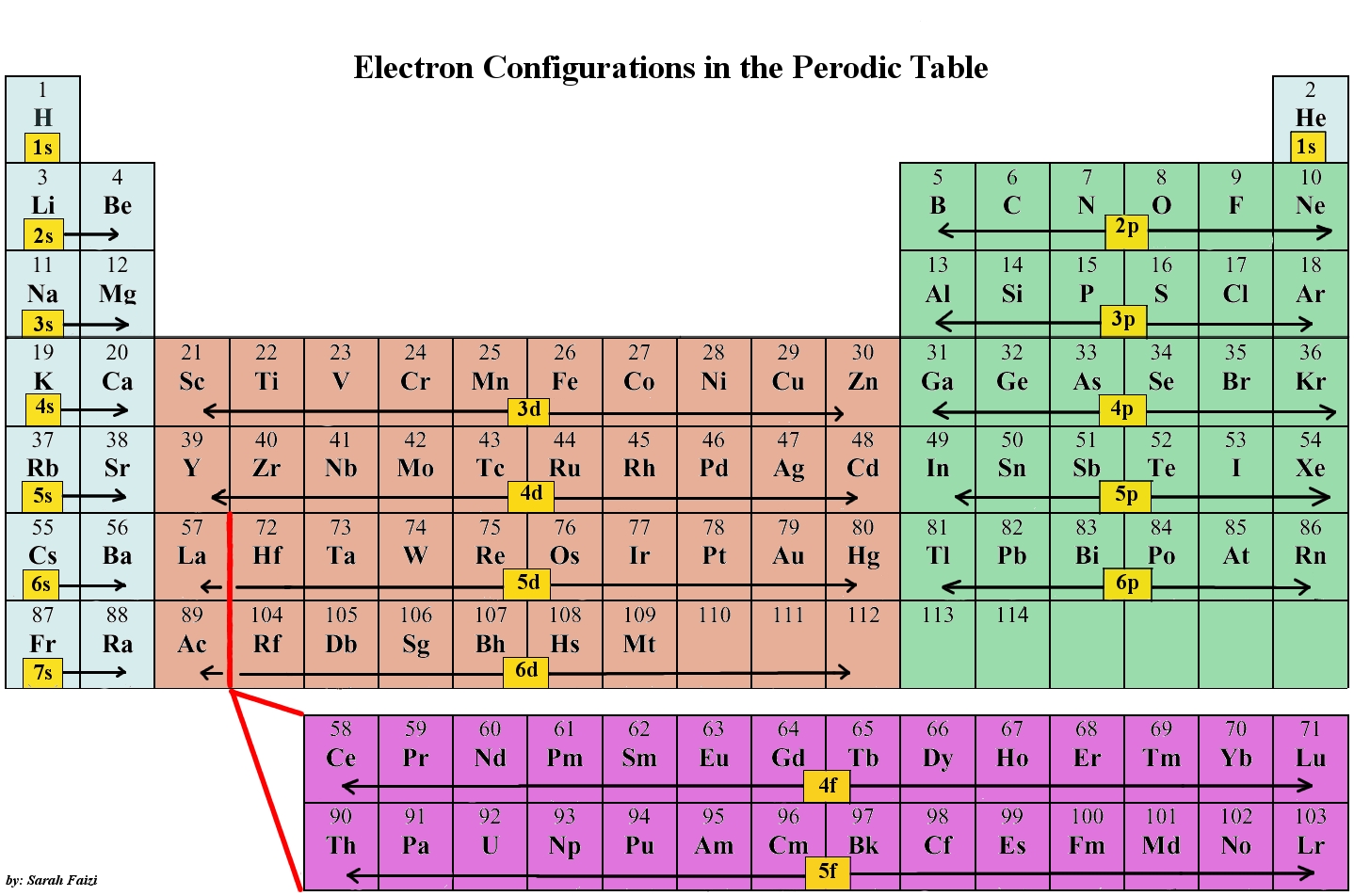
When looking at electron configuration, your fill order of electrons is:
1s
2s 2p
3s 3p 3d
4s 4p 4d 4f
5setc.
Group 1A (1), the alkali metals all end is s1. What period the element is in determines the 1st number.
Example: H ends in 1s1 (even though H is not a metal, it resides in this group because it also has one valence electron)
Li ends in 2s1 (lithium is in period 2)
Na ends in 3s1 ( sodium is in period 3)Group 2 elements (2A), the alkaline earth metals, all end in s2
Group 3A, or 13 all end their electron configurations in p1. Boron ends in 2p1.
In group 4A or 14, all elements end in p2. And so it goes.
For the transition metals, groups 3-12, there are many exceptions. The general rule is that the element's electron configuration ends in d and whatever place they are in. Scandium would end in 3d1, titanium in 3d2, etc. The transition metals are behind by one period because the d electrons are high in energy.
For the rare earth elements (the Lanthanides and Actinides), they end in f. Lots of exceptions here too. Cerium would end in 4f1, Pr in 4f2. These rare earth metals are 2 periods behind because the f electrons are even higher in energy than the d electrons.
The "s block" on the periodic table are groups 1 and 2; they end in s1 and s2.
The "p block" on the periodic table are groups 13-18 and end in p1, etc.
The "d block" on the periodic table are groups 3-12 make up the d block and the elements' electron configurations end in d.
The "f block" on the periodic table are the Lanthanide and Actinide series.
Good luck and have a great day!!
-
Electron Configurations are an organized means of documenting the placement of electrons based upon the energy levels and orbitals groupings of the periodic table.
The electron configuration for the first 10 elements
H
1s^1
He1s^2
Li1s^2 2s^1
Be1s^2 2s^2
B1s^2 2s^2 2p^1
C1s^2 2s^2 2p^2
N1s^2 2s^2 2p^3
O1s^2 2s^2 2p^4
F1s^2 2s^2 2p^5
Ne1s^2 2s^2 2p^6 The Coefficient tells us the Energy Level (Row) of the periodic table
The s or p tell us the orbital block.
The superscript tells us the number of electrons in the orbital.The s orbitals Groups 1 & 2 (columns) can hold 2 electrons
The p orbitals Groups 13 - 18 (columns) can hold 6 electrons
The d orbitals Groups 3-12 (columns) can hold 10 electrons.
The f orbitals can hold 14 electrons.Each energy level must be filled before moving up an energy level.
Each orbital group must fill before moving to the next orbital group.1s
2s 2p
3s 3p
4s 3d 4p
5s 4d 5p
6s 4f 5d 6p
7s 5f 6d 7pGermanium
#1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^2.
Germainum is in the 4th row Energy Level of the periodic table. The element is in the 2nd column of the p block, Group IVA (Column 13).I hope this was helpful.
SMARTERTEACHER