Question #c9022
1 Answer
Feb 23, 2017
Here's what I get for 1. and 2.
Explanation:
The Lewis structure of
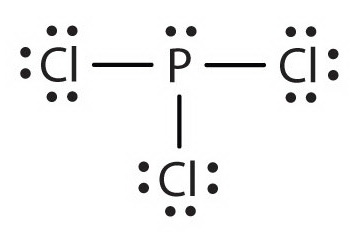
(From saylordotorg.github.io)
It has 3 bond pairs and one lone pair for a steric number (SN) of 4.
Hence its electron geometry is tetrahedral.
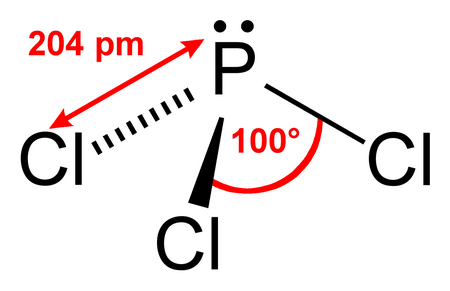
(From OpenStudy)
The molecular shape ignores the lone pair and refers only to the bonds.
Thus, the VSEPR shape of
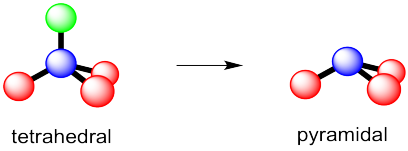
The Lewis structure of

It has 5 bonding pairs and no lone pairs, so its electron geometry and molecular geometry are the same.
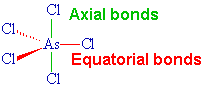
The VSEPR shape is trigonal bipyramidal.

