Why do heavier post-transition metals have smaller common oxidation states than lighter post-transition metals?
1 Answer
This has to do with the inert pair effect (i.e. with respect to the
#Se# has been known to have common oxidation states of#-2# ,#+2# ,#+4# , and#+6# .#Te# has been known to have common oxidation states of#-2# ,#+2# ,#+4# , and#+6# . We expect#+6# to be less common than#+4# .#Po# has been known to have common oxidation states of#-2# ,#+2# ,#+4# , but#color(red)(+6)# is not as common (not in bold).
These oxidation states arise due to hypothetical full transfers of SIX (FOUR) valence electrons for a
This can be explained by considering the radial density distributions of the
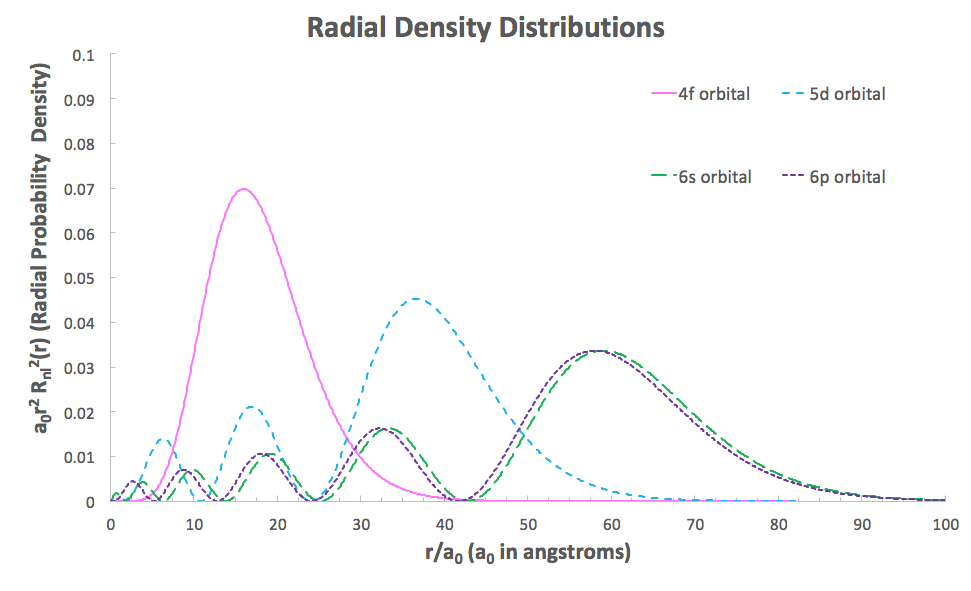
Notice how as the angular momentum of the orbital increases, the bumps near the nucleus move further away from the nucleus. This indicates decreasing ability to penetrate the core orbitals to be near the nucleus.
We can thus see the poor penetrating ability of the
That means the

