Question #d42e7
1 Answer
Mar 4, 2017
Benzene is nucleophilic.
Explanation:
The typical reaction of benzene is electrophilic aromatic substitution (
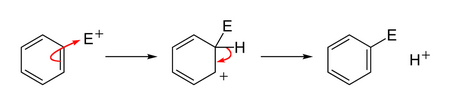 EAR
EAR
(From commons.wikimedia.org)
In the first step, we say that the electrophile
However, there is no "attack".
The two substances just collide with sufficient energy and the electrons redistribute themselves.
We could just as easily say that the benzene ring "attacks" the electrophile.
In that sense, it is acting as a nucleophile — an electron-rich species that donates an electron pair to form a bond to an electrophile.

