How do I explain differences in surface area for these alkanes?
neo-pentane vs. n-pentane
isobutane vs. butane
isobutane vs. n-propane
neo-pentane vs. n-pentane
isobutane vs. butane
isobutane vs. n-propane
1 Answer
Basically, the volume that the structure fills is roughly proportional to the amount of surface area of contact. Considering only the carbon-chain length is enough to determine the relative volumes and thus the relative surface areas.
Therefore, we can also say that for straight-chained structures, the longer the carbon chain, the more surface area it has.
Recall the following prefixes:
- meth = one carbon
- eth = two carbons
- prop = three carbons
- but = four carbons
- pent = five carbons
- hex = six carbons
- hept = . . .
- oct = . . .
This means that n-butane has four carbons, and so on. I assume "n-" is not the same as "neo". So, "neopentane" (sphere-like volume occupied, also known as "isopentane") is not the same as "n-pentane" (volume in the shape of a sandwich).
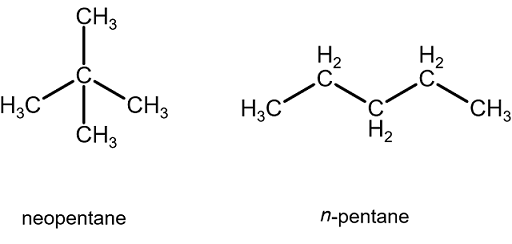
In fact, in this interpretation, "n-pentane" is generally considered the same as "pentane", for example. That would mean the only non-straight chain hydrocarbon here is isobutane (otherwise known as "neobutane").
- Isobutane has a smaller surface area than butane, because its volume is in the shape of a sphere, which minimizes surface area relative to n-butane.
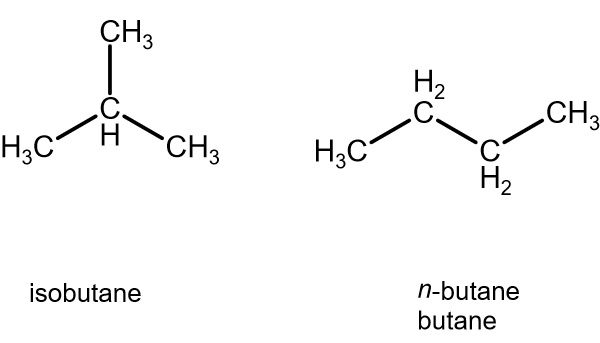
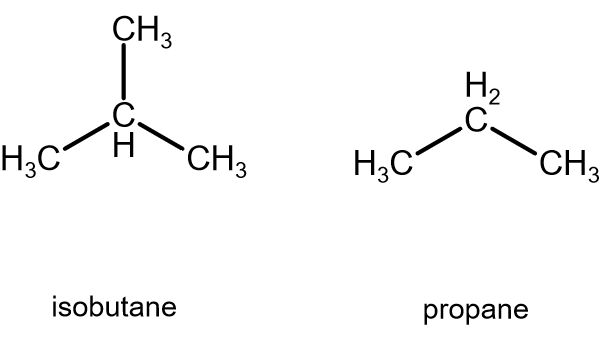
- Propane has three carbons, and has less surface area than isobutane, as it does not have a
#"CH"_3# branching off of the middle carbon.
- n-pentane has five carbons and therefore is the longest carbon chain in this group. So, it has the largest surface area as well.
Therefore, we place the ordering as:
n-pentane > n-butane > isobutane > propane
In one picture it looks like:
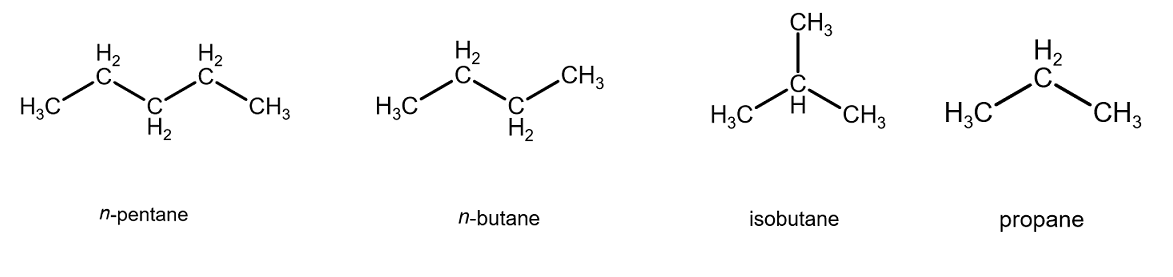
To check our work, the boiling points ought to increase with stronger intermolecular forces and thus higher surface area. So, we expect the boiling points to decrease from left to right in the above list.
The actual boiling points correspond as:
#36.1^@ "C" > -1^@ "C" > -11.7^@ "C" > -42^@ "C"#
So the above reasoning is correct.

