Question #ffc16
1 Answer
For a simple harmonic oscillator in one dimension, we have the following Hamiltonian:
#hatH = -ℏ^2/(2mu)(del^2)/(delx^2) + 1/2 kx^2 = -ℏ^2/(2mu)(del^2)/(delx^2) + 1/2 muomega^2x^2#
and the following energy (note that
#E_upsilon = ℏomega(upsilon+1/2) = hnu(upsilon+1/2)# where
#upsilon# is the vibrational quantum number,#nu# is the fundamental frequency, and#ℏ = h/(2pi)# is the reduced Planck's constant.
The zero-point energy is the energy at the bottom of the potential energy well, specifically at
#E_0 = hnu(0+1/2) = 1/2hnu = 1/2ℏomega# .
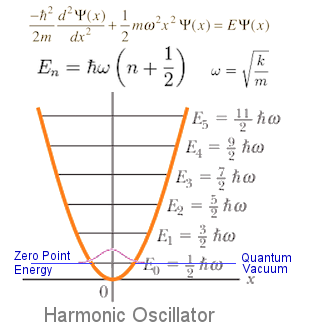
You were given that
#omega = sqrt(k/mu)# where
#mu = (m_1m_2)/(m_1+m_2) = m/2# for#"Cl"_2# ,#m# is the mass in#"kg"# (you must divide by#6.022 xx 10^(23)# to get its molar mass from#"kg/mol"# to#"kg"# if you use that), and#omega# is the angular frequency in#"s"^(-1)# .
Thus:
#color(blue)(E_0) = 1/2ℏomega = 1/2*1/(2pi)*hsqrt(k/mu)#
#= 1/(4pi)(6.626 xx 10^(-34) "J"cdot"s")sqrt("329 kg/s"^2/("0.03496885 kg/mol"/2 xx "1 mol"/(6.022 xx 10^(23) "molecules")))#
#= color(blue)(5.613 xx 10^(-21))# #color(blue)("J")#
or
#color(blue)("0.03504 eV")#
or
#color(blue)("282.59 cm"^(-1))#
A reference value from here is

