Question #c3bef
1 Answer
Mar 28, 2017
1,2-Dichlorocyclopropane has cis and trans isomers because there is no free rotation about the
Explanation:
The structures of the two isomers are shown below.
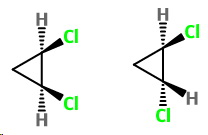
In one, both
They are both facing "up" and are on the same side of the molecule.
This is the cis isomer.
The second structure has one
They are on opposite sides of the ring, so this is the trans isomer.
It is impossible to convert one isomer into the other without breaking a
However, we are not allowed to break bonds to interconvert isomers.
Hence, the restricted rotation caused by the ring is responsible for the existence of the cis and trans isomers.

