I know reduction occurs at the cathode and oxidation occurs at the anode, but why are positive and negative terminals switched when comparing galvanic and electrolytic cells?
1 Answer
This is because the terms cathode and anode describe the function of the electrode and not its charge.
Explanation:
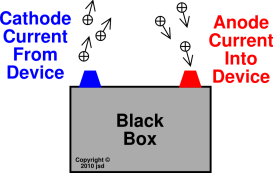
Before we go into the internal workings of the two types of cell it helps if we adopt a "black box" approach to these devices.
Conventional electric current is said to flow from the positive terminal to the negative one.

This convention was adopted in the 19th Century at the time of Michael Faraday. When the electron was later discovered and shown to have a negative charge you can see from the diagram that the electron flow is from negative to positive.
This, however, is the convention we are left with.
The ANODE of the device is the terminal where conventional current flows
This means that this is the terminal where electrons
The CATHODE of the device is the terminal where conventional current flows
This means that this is the terminal where electrons
Now lets see how this applies to an electrolytic cell and a galvanic cell.
In an electrolytic cell electrical energy is used to cause chemical change.
A good example is the electrolysis of molten lead(II) bromride:
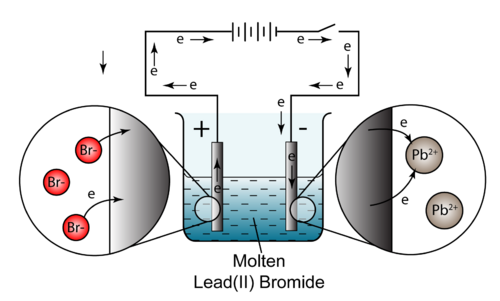
Bromide ions are attracted to the +ve electrode and discharged:
You can see from the diagram that these electrons flow into the external circuit and
By our earlier definition this makes the +ve electrode the
Lead(II) ions are attracted to the +ve electrode and discharged:
You can see from the diagram that electrons are
In a galvanic cell chemical energy is converted into electrical energy.
A good example is The Daniel Cell:

Zinc atoms go into solution as zinc ions:
So zinc forms the -ve side of the cell. You can see that these electrons flow into the external circuit and
By our earlier definition this means that the -ve zinc electrode is now the
These electrons flow round the external circuit and arrive at the copper electrode.
Here copper(II) ions pick up the electrons and a deposit of copper forms:
You can see from the diagram that electrons are
The salt bridge is there to provide electrical neutrality between the two 1/2 cells.
You should be aware that oxidation is the loss of electrons and reduction is the gain of electrons.
A good way of remembering cathode and anode in a cell is to realise that:
"Oxidation occurs at the anode"
and
"Reduction occurs at the cathode"
Care must be taken when physically labelling the electrodes on any electrical device.
In a rechargeable cell such as a lead / acid accumulator the cathode and anode will change identity depending on whether it is discharging or being charged.
In summary, the terms cathode and anode depend on the function of the electrode, not its structure.

