Question #da319
1 Answer
Apr 5, 2017
The partial pressure of the gas collected is 204.16 kPa.
Explanation:
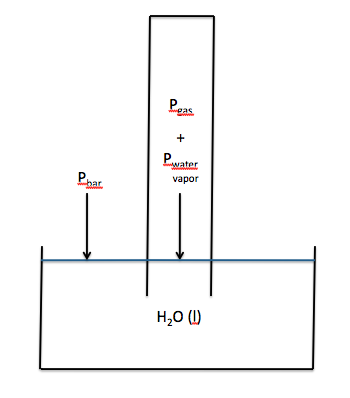
Since the gas must bubble through the water before being collected, it becomes saturated with water vapour.
When the water levels inside and outside the container are equal, the
The vapour pressure
∴

