In Experiment I, you are titrating only the "Na"_2"CO"_3, while in Experiment II you are titrating the "NaHCO"_3 formed in Experiment I plus what was present in the original mixture.
Experiment I
 Experiment I
Experiment I
In Experiment I, you are titrating the mixture of "Na"_2"CO"_3 and "NaHCO"_3 to a phenolphthalein end-point.
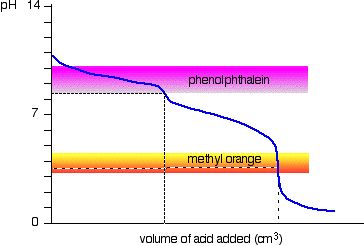
Phenolphthalein changes colour around pH 9.
The titration curve for "Na"_2"CO"_3 looks like this:
 www.chemguide.co.uk
www.chemguide.co.uk
At the endpoint, you will have neutralized only the "Na"_2"CO"_3.
You will not yet have neutralized any "NaHCO"_3, so the milliequivalents of "NaHCO"_3 neutralized are zero.
Experiment II
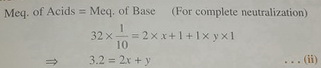 Experiment II
Experiment II
In Experiment II, you are titrating both the "NaHCO"_3 that was formed in Experiment I and the "NaHCO"_3 that was present in the beginning.
Thus, the milliequivalents of base ("NaHCO"_3) are those you formed in Experiment I plus those that were initially present.
 Experiment I
Experiment I  www.chemguide.co.uk
www.chemguide.co.uk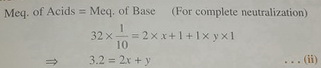 Experiment II
Experiment II 
