Why is the tosylate anion a good leaving group?
1 Answer
Apr 12, 2017
Because God wanted it that way..........
Explanation:
The standard answer to your question is that the
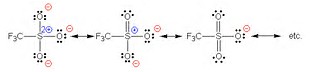 chemgapedia.de
chemgapedia.de
The illustration depicts the anion of trifluoromethane sulfonic acid, (whose anion is another good leaving group!), and of course this is similar to the resonance stabilization of the tosylate ion, where the anionic charge can also by distributed to the aryl ring.

