How should the ionization of metals and non-metals compare? Which process should require more energy?
1 Answer
As we face the Table, non-metals come from the right of the Periodic Table............
Explanation:
And metals come from the left of the Table (as we face it......).
Now incomplete electronic shells shield the nuclear charge VERY imperfectly. The result? Across a given Period, atomic radii (which of course is the radius of the valence electronic shell) STRONGLY decrease. And look at how the atomic radius decreases across a given Period, across the Table.
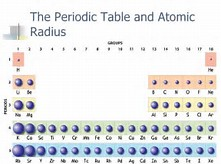
The atomic radius of the Noble Gases, are the smallest, for the reason that nuclear charge wins across the Period, and contracts the atomic radius by reason of the increased nuclear charge.
And as we descend a Period, the nuclear charge is somewhat shielded by a full valence shell. The next valence shell is farther removed from the nucleus, and the process begins again. This contest between nuclear charge, and shielding by other electrons, underlies the structure of the modern Periodic Table.
As chemists, as physical scientists, we should out some data to inform our argument. And consider the ionization enthalpies of the elements across the Period,
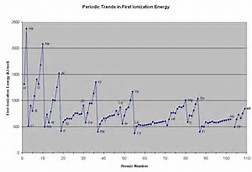
The LARGE ionization enthalpies of say, oxygen, and fluorine, and argon, are good evidence of the effects of increasing the nuclear charge,
And so, finally, to address your question. These data suggest a priori that metal ions, which have unfilled valence shells should be easier to ionize than non-metals, which come from the right of the Periodic Table, and tend to have high effective nuclear charge. And indeed they are: metals thus tend to lose loosely bound valence electrons to form
On the other hand, towards the right of the Table, where lie the non-metals, these species tend to be oxidizing (certainly oxygen and flurorine are!), and their high nuclear charge is the reason for this tendency.

