If the forward reaction is exothermic, why is the reverse reaction endothermic?
1 Answer
Apr 24, 2017
Because, by necessity, the backward rate of activation is equal to the FORWARD activation energy PLUS the ENERGY RELEASED by the exothermic reaction.........
Explanation:
This is best illustrated by interrogating the diagram.......
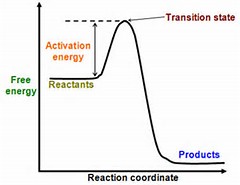
The forward rxn (left to right as you face it) was clearly EXOTHERMIC. To go from right to left, we have to input energy into the system: the energy that was released from the forward reaction PLUS the original energy of activation. I think it is fairly clear from such a graph as to the energy requirements of the reaction.

