Question #17b01
1 Answer
Here's what I got.
Explanation:
You're dealing with the beta minus decay of neptunium-238.
In beta minus decay, a neutron located inside the nucleus of a radioactive nuclide is being converted to a proton. The process releases an electron, also called a beta particle, and an electron antineutrino,
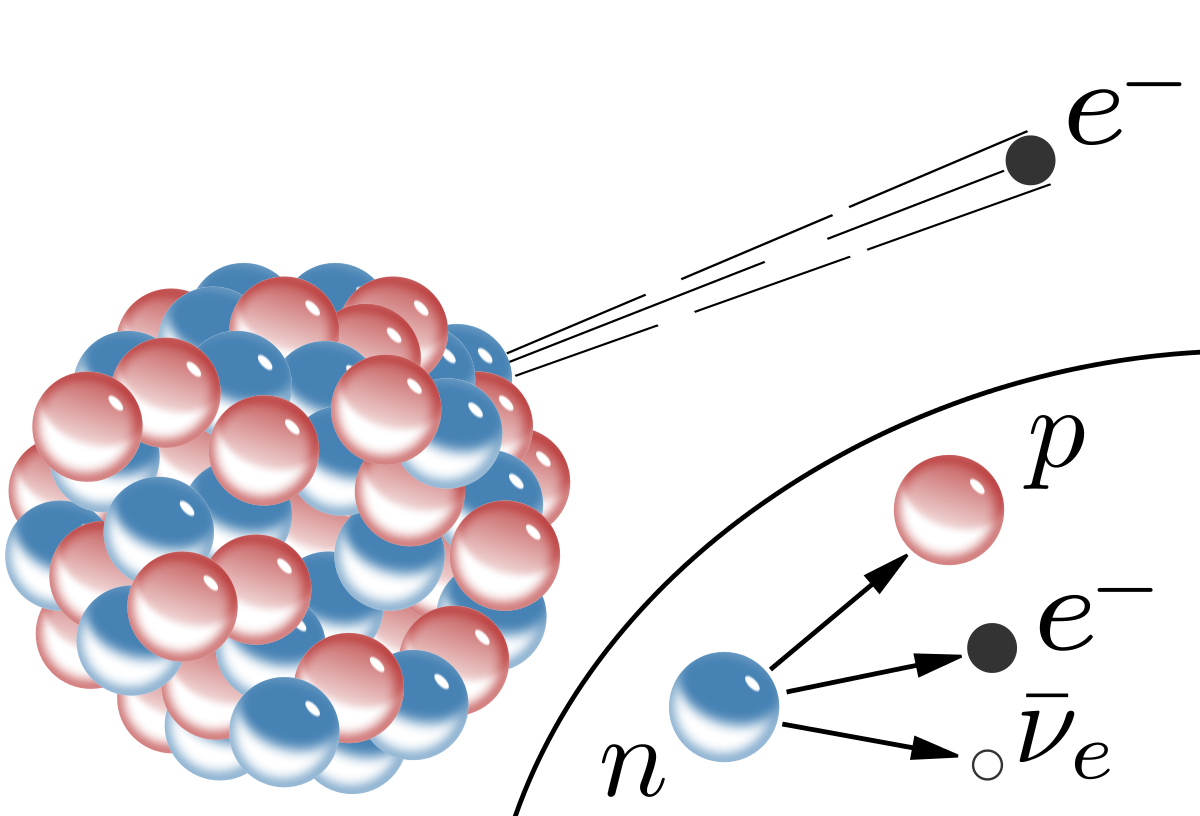
So, you know that you're starting with
#""_ (color(white)(1)color(darkgreen)(93))^color(blue)(238)"Np" -> ""_ color(darkgreen)(Z)^color(blue)(A)"?" + ""_ color(darkgreen)(-1)^(color(white)(-)color(blue)(0))"e" + bar(nu)_e#
Your goal here is to figure out the values of
As you know, mass and charge must be conserved in nuclear equations. This means that you can write
#color(darkgreen)(93 = Z + (-1)) -># conservation of charge
#color(blue)(238 = A + 0) color(white)(aaa)-># conservation of mass
You can thus say that
#Z = 93 + 1 = 94" "# and#" "A = 238#
Grab a Periodic Table and look for the element that follows neptunium. You'll find that this element is plutonium,
You can now complete the nuclear equation that describes the beta minus decay of neptunium-238
#""_ (color(white)(1)color(darkgreen)(93))^color(blue)(238)"Np" -> ""_ (color(white)(1)color(darkgreen)(94))^color(blue)(238)"Pu" + ""_ color(darkgreen)(-1)^(color(white)(-)color(blue)(0))"e" + bar(nu)_e#

