Question #ed671
1 Answer
Never!
Explanation:
The thing to remember about chemical formulas is that they can never use fractional subscripts. Ever.
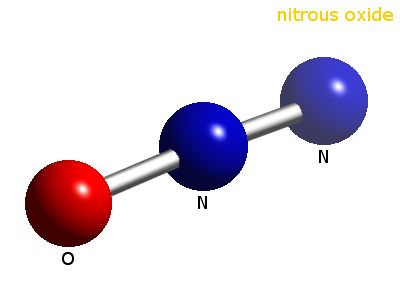
In this case, the molecular formula for nitrogen oxide, also known as dinitrogen monoxide, is
- two atoms of nitrogen,
#2 xx "N"# - one atom of oxygen,
#1 xx "O"#
Now, you can divide the formula by
- one atom of nitrogen,
#(2 xx "N")/2 = 1 xx "N"# - one half of an atom of oxygen,
#(1 xx "O")/2 = 1/2 xx "O"#
That is not possible because you cannot really have a half of an atom of oxygen, or of any atom, for that matter.
In other words, the chemical formula must make sense at the level of the atom, which is why it cannot use fractional subscripts.
#"no fractions of an atom = no fractional subscripts"#
In other words
#"subscripts = whole numbers"#
Moreover, you also need to keep in mind that even if you can simplify the subscripts to get whole numbers, you cannot do that without changing the nature of the compound.
For example,
- two atoms of nitrogen,
#2 xx "N"# - four atoms of oxygen,
#4 xx "O"#
You can divide the formula by
- one atom of nitrogen,
#(2 xx "N")/2 = 1 xx "N"# - two atoms of oxygen,
#(4 xx "O")/2 = 2 xx "O"#
But
#"N"_2"O"_4 != "NO"_2#

