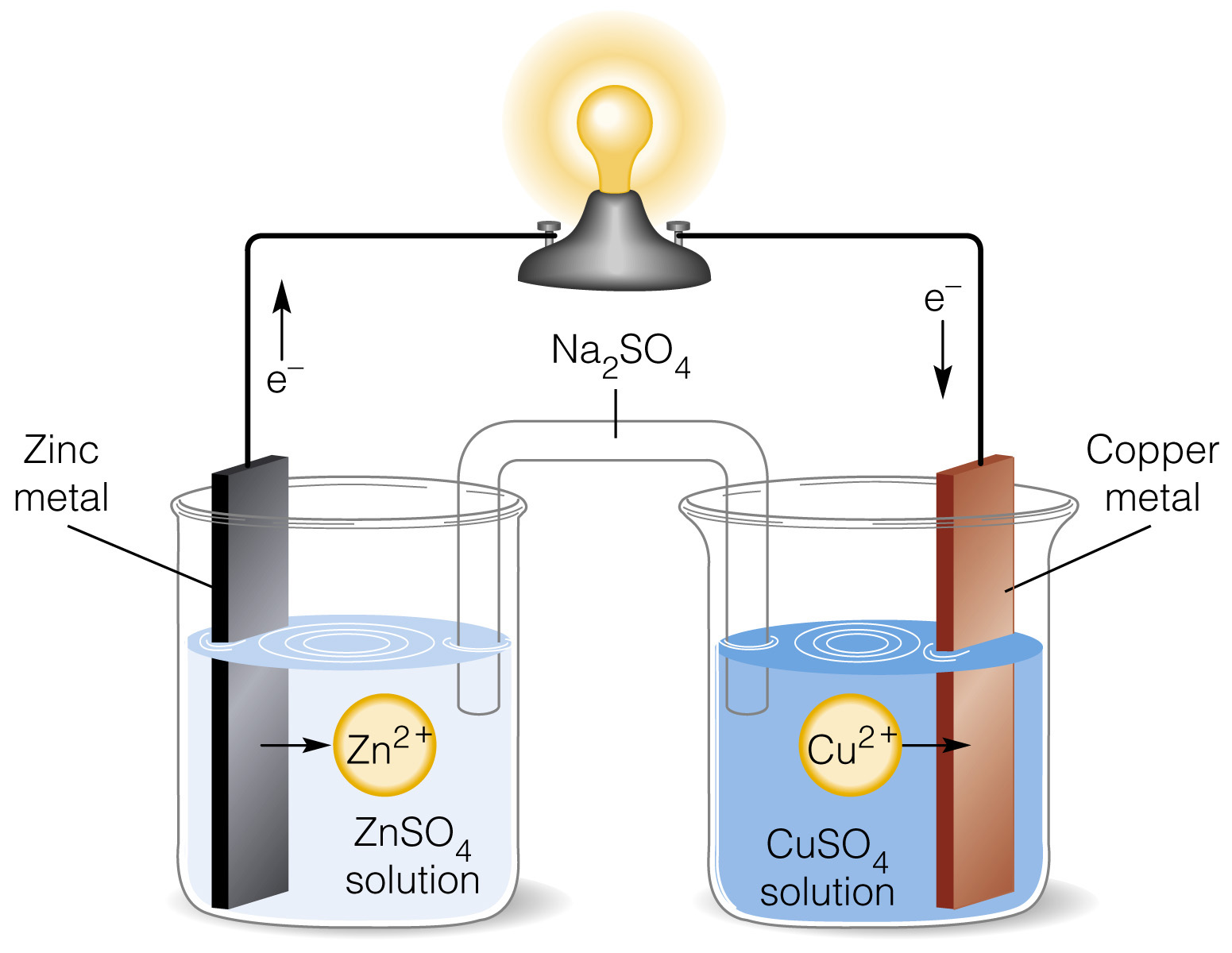
The arrangements in Daniel cell
 google
google
Anode reaction
Zn(s)->Zn^(2+)(aq)+2e^-
Cathode reaction
Cu^(2+)(aq)+2e^(-) ->Cu(s)
Overall reaction
Zn(s)+Cu^(2+)(aq)->Cu(s)+Zn^(2+)(aq)
The cell notation
Zn(s)|Zn^(2+) ||Cu^(2+)|Cu(s)
The Cell Emf by Nernst equation
E_(cell)=E_(cell)^@-(RT)/(nF)xx([Zn^(2+)])/([Cu^(2+)])......[1]
If we add sodium sulphide (Na_2S) to cupper sulphate in daniel cell, it interacts with Cu^(2+) in solution and lowers the concentration of Cu^(2+) by precipitating it as insoluble CuS(s) as per the following equation.
Cu^(2+)(aq)+Na_2S->CuS(s)darr+2Na^+
As a result the ratio ([Zn^(2+)])/([Cu^(2+)]) in equation [1] increases and this decreases the E_(cell) to a great extent.
google

