Question #e64fe
1 Answer
May 24, 2017
NO (not in our universe, anyway)
Explanation:
double and triple bonds bond using pi bonds,
these sit above and below the axis of the sigma bond (sigma bonds are regular single covalent bonds)
for a double bond these pi bonds sit in above and below the axis
for instance,
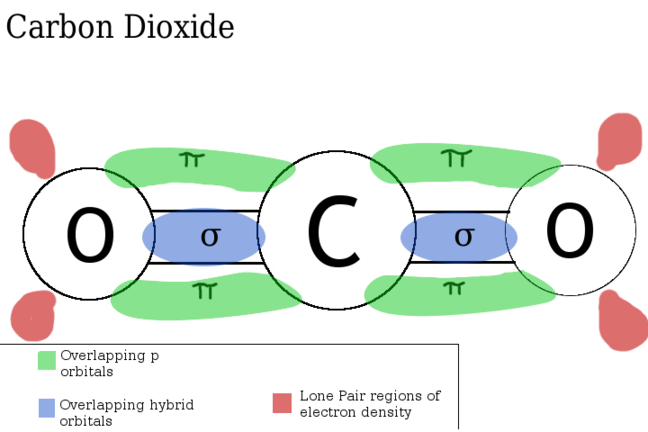
for a triple bond bond the pi bonds sit above and below AND in-front and behind the axis,
for instance,
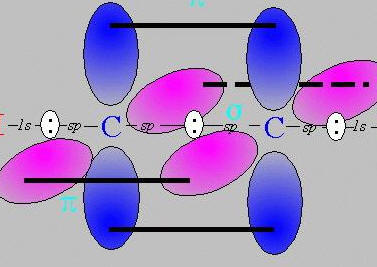
As we live in a 3-D universe (3 dimensions of space, ignoring time dimension), there are no other axes to use for any other pi bonds.
therefore a triple bond is the most you can get.
therefore no quadruple bonds. =(

