Which of the following can hydrogen-bond with itself?
(a) butane
(b) acetone
(c) acetic acid
1 Answer
Should be
While it's true that acetone,
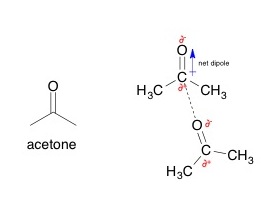 https://upload.wikimedia.org/
https://upload.wikimedia.org/
Acetic acid,
 https://upload.wikimedia.org/
https://upload.wikimedia.org/
By recalling the relative intermolecular forces and their strengths...
London Dispersion
< Dipole-Dipole< H-"bonding"< Ion-Dipole< Ion Pair
...and recalling that as intermolecular force strengths increase, molecules stick together more and vaporize less easily (corresponding to a higher boiling point), we would then have that:
- since butane,
"CH"_3"CH"_2"CH"_2"CH"_3 , only has London Dispersion (being a hydrocarbon, a nonpolar molecule!), it has the lowest boiling point. - since acetone has dipole-dipole interactions with ITSELF, it has the second-highest boiling point.
- since acetic acid hydrogen-bonds with ITSELF, it has the highest boiling point.
And we can't go without the data. From a quick Google search:
-1^@ "C" for butane
56^@ "C" for acetone
118.1^@ "C" for acetic acid
which follows the trend predicted above. Hence, the answer is

