Question #fdc63
1 Answer
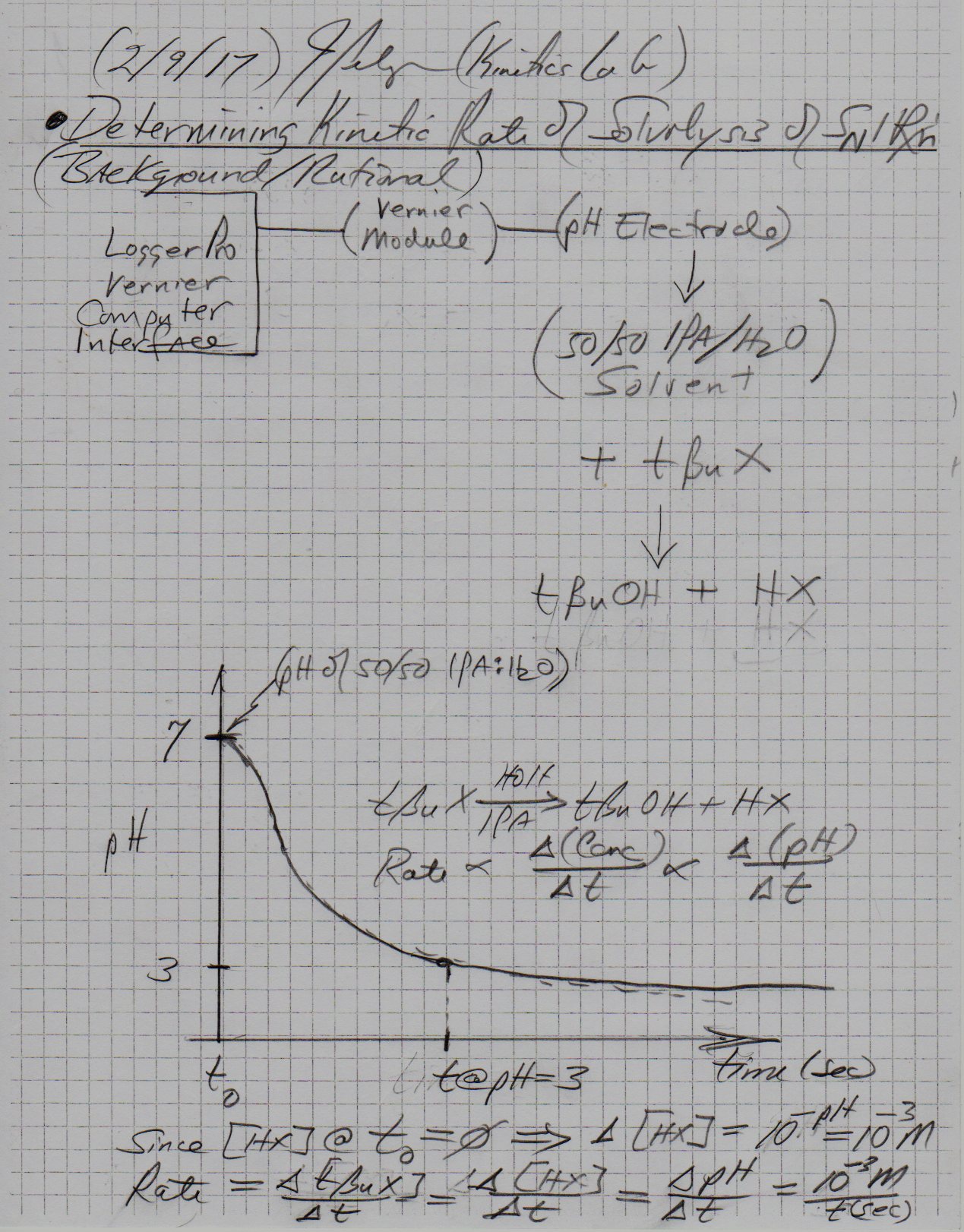
The kinetics of solvolysis of t-Butyl Halides in a 50/50 IPA:HOH solvent system can studied using change in pH as a function of time.
t-BuX + HOH => t-BuOH + HX can be monitored with a pH meter and give very accurate results. One such experimental set-up is to use a Logger Pro - Vernier Computer interface with a pH electrode to monitor change in pH as a function of time. The data collected is generated in real time with addition of specific neat quantities of t-BuX into the 50/50 IPA:HOH solvent system.
The following is an excerpt from a lab journal showing the rational - background foundation for Study of Kinetics of Solvolysis of t-Butyl Halides in Binary Alcohol/Water Solvents.
The lab is quite extensive, but this excerpt will answer your question.