Which of these is the most stable carbocation and why?
(a) benzyl
(b) diphenylmethyl
(c) triphenylmethyl
(a) benzyl
(b) diphenylmethyl
(c) triphenylmethyl
1 Answer
Jun 7, 2017
The most stable carbocation is c) triphenylmethyl.
Explanation:
All three carbocations are more stable than alkyl cations because the positive charge can be delocalized into the aromatic ring.
(a) Benzyl cation
The benzyl cation has four resonance contributors: one on the exocyclic carbon and three in the ring.
Benzyl
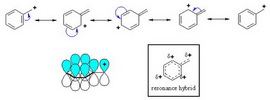
(From jahschem.wikispaces.com)
(b) Diphenylmethyl cation
The second aromatic ring provides three more resonance contributors, so there are now seven resonance structures..
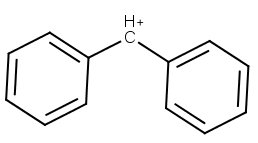
Triphenylmethyl cation
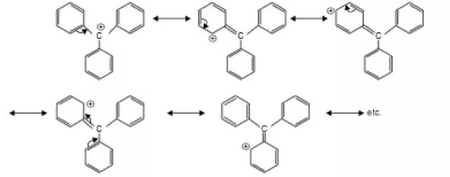
(From quora.com)
The triphenylmethyl cation has ten resonance contributors, so it is the most stable carbocation.

