To determine which of these has a permanent dipole, we must first draw the Lewis structures for each compound:
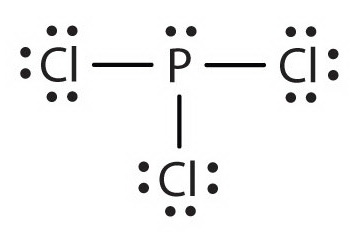
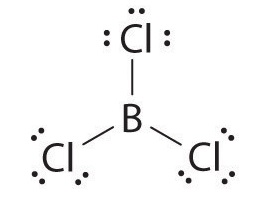
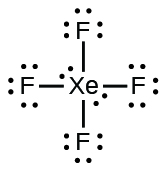
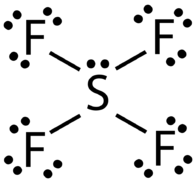
A molecule has a permanent dipole moment if it contains unequally distributed electrons throughout the molecule.
In #"BCl"_3#, the geometry is trigonal planar, and the electrons are shared evenly outward from the central #"B"# atom. It therefore has no dipole moment.
In #"XeF"_4#, the electron distribution is symmetrical (the two lone pairs "cancel" each other out, and the four bonding pairs are distributed evenly in two-dimensional space), so it also has no dipole moment
For #"PCl"_3# and #"SF"_4#, the single lone pair about the central atom indicates that the electrons are not shared equally across the molecule (no matter how many bonding pairs surround the central atom), so both of these have a permanent dipole.




