How does the solubility of aldehydes and ketones change in water as the number of carbon atoms changes?
1 Answer
The solubilities of both aldehydes and ketones in water decrease as the number of carbon atoms increases.
Explanation:
However, alkyl groups are electron-donating groups, so ketones are more polar than aldehydes.
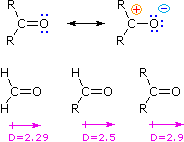
Thus, ketones are slightly more soluble than aldehydes with the same number of carbon atoms.
Here's a table of the solubilities
The dividing line for "soluble" is about four carbon atoms for aldehydes and five carbon atoms for ketones.


