How will the density of mercury change when it is heated?
1 Answer
Well, to know that we should look at its phase diagram (though we expect expansion of the liquid).
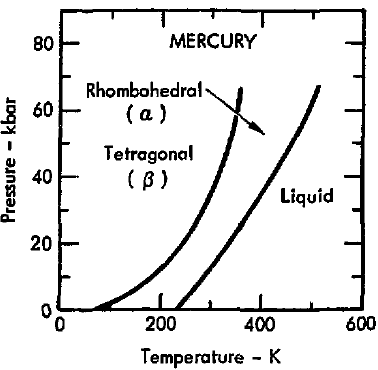
You didn't specify starting from what temperature pressure, but we assume
An ordinary temperature increase (
#=> V = m/D# ,where
#m# is mass and#D# is density.
One can see then that the slight increase in volume decreases the density slightly.

