Question #19789
2 Answers
Jun 23, 2017
Explanation:
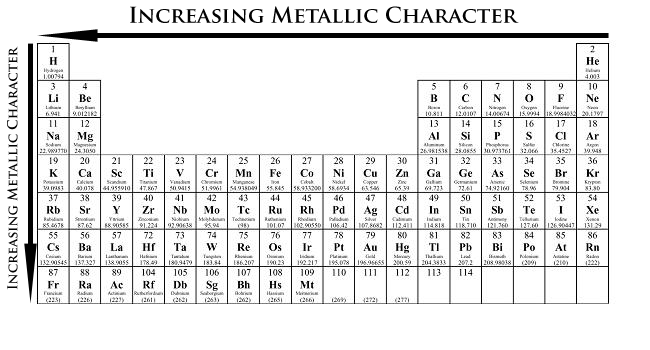
Metallic character increases going down and to the left.
Jun 23, 2017
Rb, Ga, Al, Si, P, N
Explanation:
The periodic trend of metallic character increases from left to right, and from top to bottom on the periodic table.
Rb is a group 1/IA metal on the far left side of the periodic table, so it is much more metallic than the other elements listed in the question. Al and Ga are in group 13/IIIA. Since Ga is below Al, it has a greater metallic character than Al. Si is further to the right in group 14/IVA, so it is less metallic than Al, but it is more metallic than P and N. P and N are in group 15/VA. P is below N, so P would have the greater metallic property.