Question #72df6
1 Answer
The particles will move faster around in the available space.
Explanation:
If the temperature is increased, it means that the molecules will move (on average) with a higher speed. This means that the molecules have a higher kinetic energy:
Kinetic energy =
If we warm up a solution, we actually transfer energy into the molecules. This energy can be used as kinetic energy. Since the mass of the molecules doesn't change in this process, the velocity must increase. Therefore, the molecules will move faster around.
If a molecule has sufficiently enough speed, it could escape the solution and then it is called vapour or gas. This point where that happens is characteristic for a solution and called its boiling point.
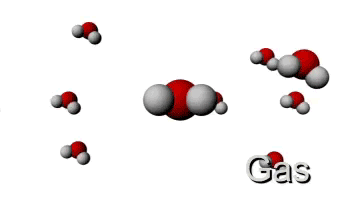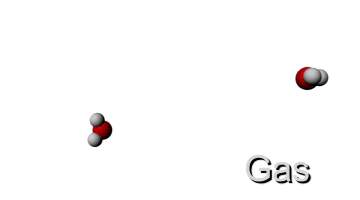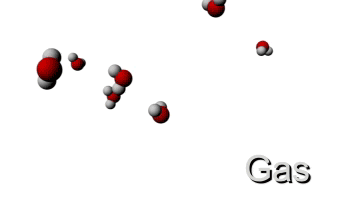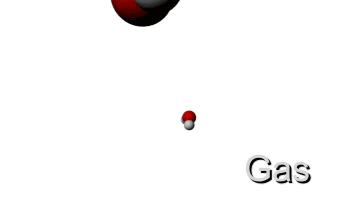
Now let's see what happens if we give the molecule more energy by increasing the temperature. This means that the kinetic energy will go up and since the mass stays the same, the velocity must increase too. Therefore the molecule will have a higher velocity when the temperature is increased. This is visualised below.
HIGH TEMPERATURE
 http://www.ergopedia.com/ergoweb/pedagogy_technology.php
http://www.ergopedia.com/ergoweb/pedagogy_technology.php
LOW TEMPERATURE
 i.imgur.com
i.imgur.com
(same source as above)
