Question #db16f
1 Answer
Jul 4, 2017
Explanation:
All you have to do here is to use the ideal gas law equation, which looks like this
#color(blue)(ul(color(black)(PV = nRT)))#
Here
#P# is the pressure of the gas#V# is the volume it occupies#n# is the number of moles of gas present in the sample#R# is the universal gas constant, equal to#0.0821("atm L")/("mol K")# #T# is the absolute temperature of the gas
Notice that the problem provides the temperature of the gas in degrees Celsius, so make sure to convert this to Kelvin!
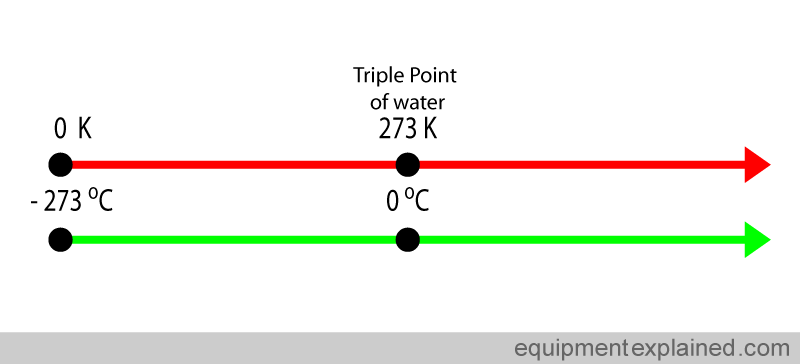
Rearrange the equation to solve for
#PV = nRT implies n = (PV)/(RT)#
Plug in your values to find
#n = (11.2 color(red)(cancel(color(black)("atm"))) * 0.24 color(red)(cancel(color(black)("L"))))/(0.0821 (color(red)(cancel(color(black)("atm"))) * color(red)(cancel(color(black)("L"))))/("mol" * color(red)(cancel(color(black)("K")))) * (273.15 + 12)color(red)(cancel(color(black)("K"))))#
#n = color(darkgreen)(ul(color(black)("0.11 moles"))#
The answer is rounded to two sig figs.

