Question #f2414
1 Answer
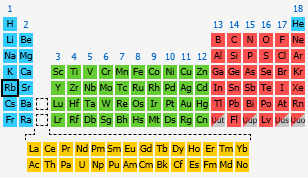
Grab a Periodic Table!
Explanation:
Your tool of choice here will be good old Periodic Table of Elements, so grab one and look for rubidium,
You should find this element in period
Now, you should know that the principal quantum number,
In your case, rubidium is located in period
#n = color(red)(5)#
You should also know that the Periodic Table is divided into blocks.
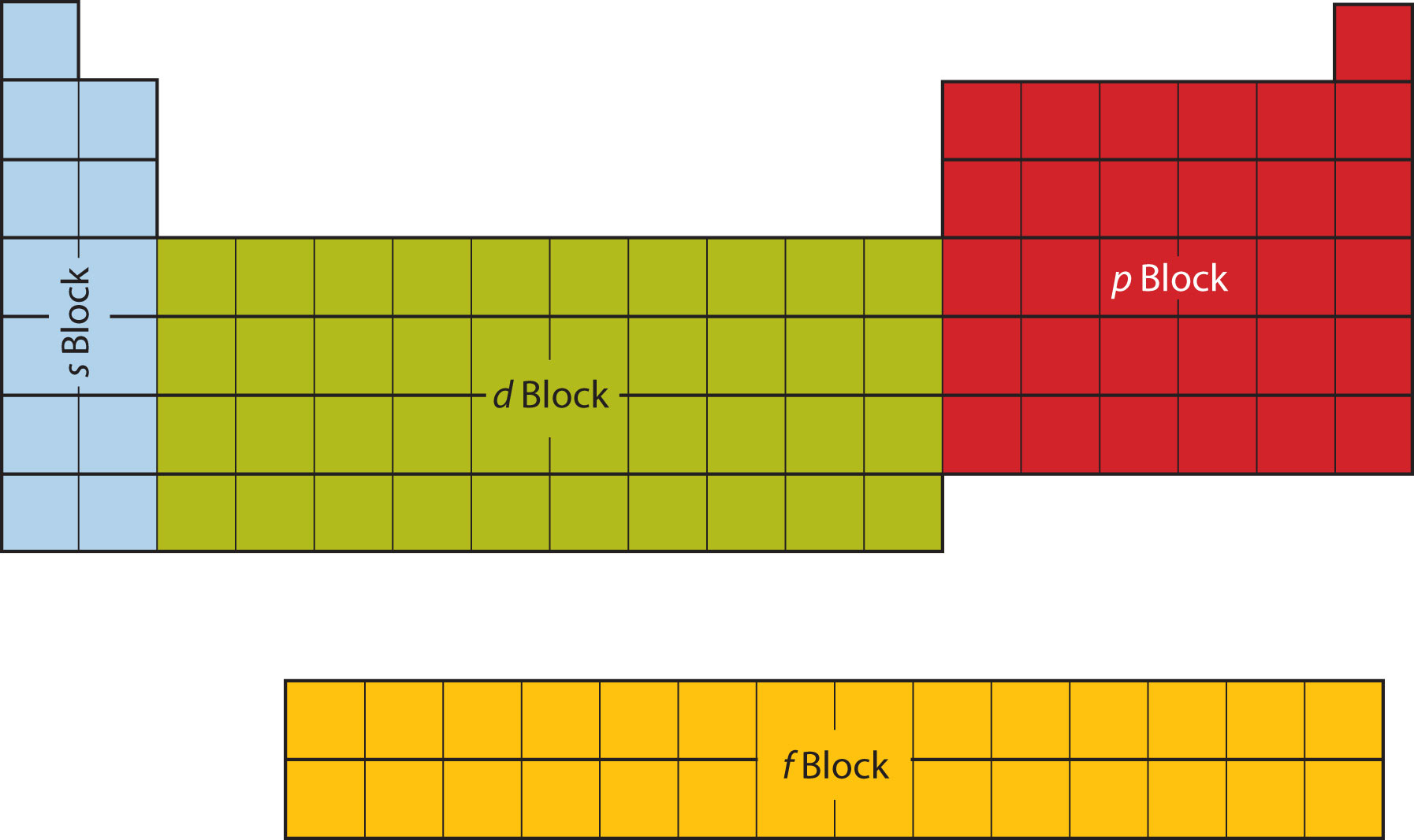
As you can see, the elements located in groups
This means that their outermost electron(s) are located in the
You will have
#l = 0 -># the#s# subshell#l=1 -># the#p# subshell#l = 2 -># the#d# subshell#vdots#
and so on. In your case, rubidium is located in group
#l = color(blue)(0) -># the#s# subshell regardless of the value of#n#
You now have
#n = color(red)(5), l = color(blue)(0)#
The third quantum number in the set is the magnetic quantum number,
Since the magnetic quantum number can only take one possible value for the
#l = 0 implies m_l = 0#
you can say that you now have
#n = color(red)(5), l = color(blue)(0), m_l = 0#
Finally, the spin quantum number,
#m_s = {-1/2, +1/2}#
By convention, every electron that occupies an empty orbital, i.e. an electron that is present in an orbital by itself (an unpaired electron), is taken to have spin-up, which corresponds to
#m_2 = +1/2 -># an electron that has spin-up
This means that the full set of quantum numbers that describes the outermost electron of rubidium is
#n = 5, l =0, m_l = 0, m_s = +1/2#