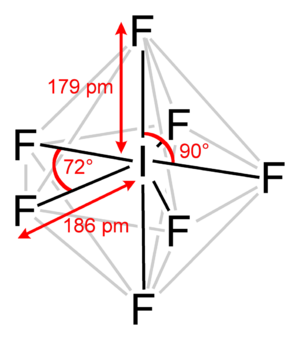
The interhalogen follows the normal application of VESPER, valence shell electron pair repulsion theory. The central atom, which is almost always the least electronegative atom......is iodine.
The most stable electronic arrangement to accommodate 7 bonding electron pairs is as a pentagonal bipyramid as shown.....
 useruploads.socratic.org)
useruploads.socratic.org)
Now such a geometry is conformationally mobile, and while I have never done the experiment, in the 19F{1H} NMR spectrum at room temperature, we would probably see the one signal due to exchange between the axial and equatorial fluorines in a mechanism similar to Berry pseudorotation observed for PF5.... And should the temperature be lowered to below a certain temperature, we would see the absorption decoalesce to give 2 signals in a 2:5 ratio (in a triplet and a sextet), the one representing the axial fluorine nuclei, and the other representing the fluorine atoms in the plane.
 useruploads.socratic.org)
useruploads.socratic.org) 
