Why is the election affinity of beryllium negative?
1 Answer
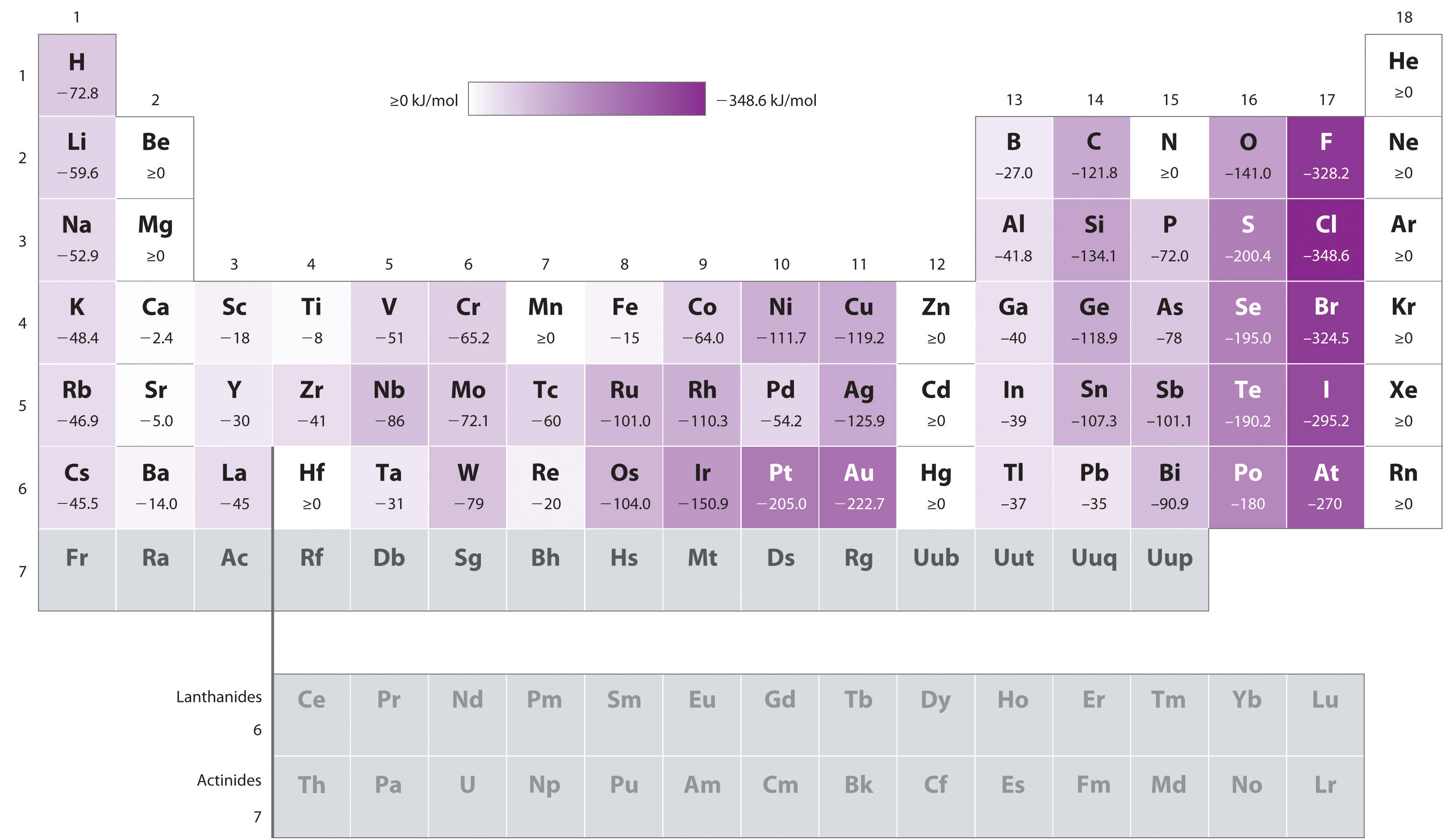
Well, beryllium has a POSITIVE electron affinity, because it gets less stable when we try to fill the next orbitals, the
 https://files.mtstatic.com/
https://files.mtstatic.com/
The electron affinity is the change in energy due to adding a new electron to an atom or molecule. The to-scale electronic structure of beryllium is:
underbrace(ul(color(white)(uarr darr))" "ul(color(white)(uarr darr))" "ul(color(white)(uarr darr)))
" "" "" "color(white)(.)2p
" "
" "
" "
" "
ul(uarr darr)
color(white)(.)2s
" "
" "
" "
" "
" "
" "
" "
" "
" "
" "
" "
" "
" "
" "
" "
" "
ul(uarr darr)
color(white)(.)1s
And so, it takes energy input in order to place a new electron into a

