A 20*g mass of phosphoric acid dissolved in 250.0*mL aqueous solution, reaches a stoichiometric end-point with WHAT volume of NaOH(aq) at 0.120*mol*L^-1 concentration?
1 Answer
Jul 31, 2017
We need (i) a stoichiometric equation.....
Explanation:
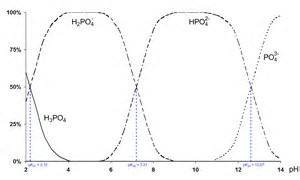
Note a stoichiometric endpoint is reached upon the addition of 2 equiv of base.
And (ii) we need equivalent quantitites of
Phosphoric acid is a diacid in aqueous solution.
 commons.wikimedia.org
commons.wikimedia.org

