What is the oxidation number of the ipso carbon in #HC(=O)O^(-)#?
2 Answers
I make it
Explanation:
We gots
And so
Note that for acetate ion or acetic acid, the corresponding oxidation number is
For general rules on the assignment of oxidation numbers see here.
+2
Explanation:
The Lewis Dot Structure analysis may be the best visual.
When a Lewis dot structure of a molecule is available, the oxidation states may be assigned by computing the difference between the number of valence electrons that a neutral atom of that element would have and the number of electrons that "belong" to it in the Lewis structure.
For purposes of computing oxidation states, electrons in a bond between atoms of different elements belong to the more electronegative atom; electrons in a bond between atoms of the same element are split equally, and electrons in alone pair belong only to the atom with the lone pair.
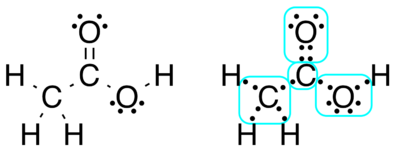
From https://en.wikipedia.org/wiki/Oxidation_state
The methyl group carbon atom has six valence electrons from its bonds to the hydrogen atoms because carbon is more electronegative than hydrogen. Also, one electron is gained from its bond with the other carbon atom because the electron pair in the C−C bond is split equally.
It therefore has a total of seven electrons, whereas a neutral carbon atom would have four valence electrons because carbon is in group 14 of the periodic table. The difference, 4 − 7 = −3, is the oxidation state of that carbon atom. That is, if it is assumed that all the bonds were 100% ionic (which in fact they are not), the carbon would be described as C3−
.


