How do you name [Co(en)2CO3]Br?
1 Answer
Assuming you mean...
#["Co"("en")_2"CO"_3]"Br"# ,
this is a carbonatobis(ethylenediamine)cobalt(III) bromide isomer (either
#"en"# , ethylenediamine, is a neutral ligand. The complex name warrants a new kind of prefix, bis, to indicate that there are two of them. If there were three, one would use tris.#"CO"_3^(2-)# is the carbonato ligand.#"Br"^(-)# in the outer coordination sphere implies that the inner coordination sphere is a#+1# charge overall.- Thus, the oxidation state of cobalt is
#+"III"# ...
That gives
There are, however, two isomers that you should be aware of... the
There are two isomers for this complex:
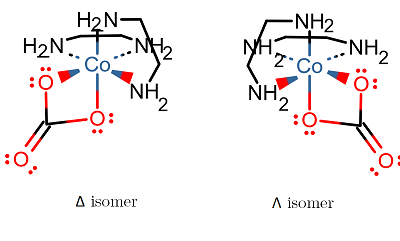
There is not a third, where the carbonato is "bonded" trans, because it is a bidentate ligand. It would be too strained to bond across a
-
#Delta# means that of two identical bidentate ligands, with one of them as a rear fin, the other is a right-handed fin. -
#Lambda# means that of two identical bidentate ligands, with one of them as a rear fin, the other is a left-handed fin.
These are optical isomers, and are non-superimposable mirror images of each other, basically a complicated enantiomer.

