Explain associative interchange reactions? What is the mechanism?
1 Answer
Associative interchange, or
- the departing ligand
#X# is assumed to have a fairly weak bond with the metal#M# that the incoming#Y# ligand can easily disrupt. That is,#Y# is a good nucleophile and the#M-X# bond is fairly weak. - the departing ligand
#X# and incoming#Y# basically swap by the time the reaction is over.
However, there are two accepted variations of this mechanism that I know of.
Consider a square planar complex
The regular process is described in
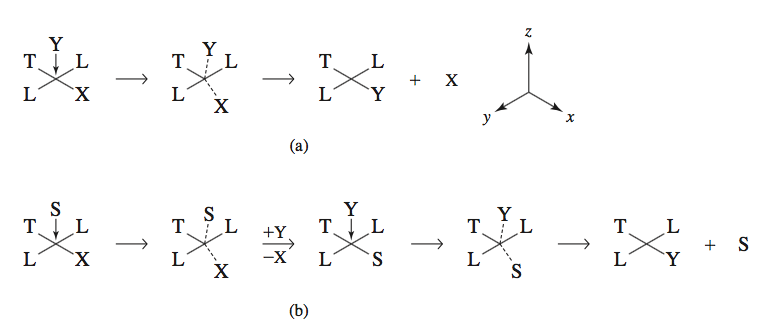
Typically, a 5-coordinate transition state forms, but a 6-coordinate transition state is also known, with assistance from solvent (Inorganic Chemistry, Miessler et al., pg. 458).
A two-term general rate law is frequently used to describe a square-planar substitution process:
#r(t) = overbrace(k_1["Cplx"])^((b)) + overbrace(k_2["Cplx"][Y])^((a))# where:
-
the
#k_1# portion describes the pseudo-first-order solvent-assisted process. -
the
#k_2# portion describes the regular, second-order association process. The complex referred to pertains to#"MXL"_2"T"# .
Referring to the diagram above:
-
In
#(a)# ,#Y# comes in and forms a 5-coordinate transition state, and displaces#X# with assistance from#T# due to the trans effect. See here for a discussion on the trans effect. As both the complex and#Y# participate in one step, this is second order overall. -
In
#(b)# , the solvent#S# comes in and displaces#X# first. This step is presumed slow, while the second step, in which#Y# displaces the solvent#S# , is presumed fast.
Since the solvent is presumed in large excess, the order of
#S# is approximately zero, and thus, the solvent-assisted mechanism is pseudo-first-order in the complex.

