Question #24a9d
1 Answer
Needless to say, they are different. The molecular weight is the sum of the atomic weight.
Explanation:
[What is the atomic mass?]
The atomic mass is the average mass of an element. It is the relative mass compared to
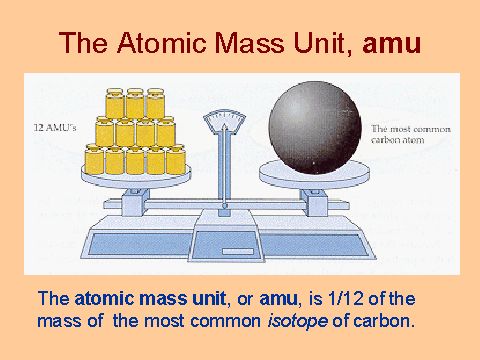
Example:
(http://www.sisweb.com/referenc/source/exactmas.htm)
The abundance(%) for
Therefore, the atomic mass of carbon is
[What is the molecular mass?]
The molecular mass is the average relative mass of an molecule, compared to
For example, the atomic mass of hydrogen is
The molecular mass of methane(

