Question #1b269
1 Answer
Sep 15, 2017
Here's why that is the case.
Explanation:
Your quantum number set cannot describe an electron in an atom because the value of the magnetic quantum number,
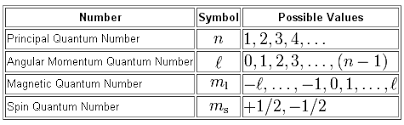
In your case, you have
#(color(red)(3), color(blue)(2), color(purple)(3), -1/2)#
The electron is said to be located on the third energy level, which has
#l = color(blue)(2)#
For the
#m_l = {-2, -1, 0, 1, 2}#
As you can see,

