Question #51981
1 Answer
Explanation:
For starters, grab a Periodic Table and look for silicon,
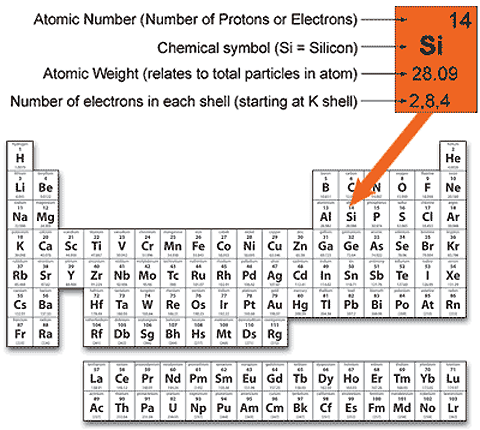
Now, notice that silicon has an atomic number equal to
In your case, you need to find the set of quantum numbers that describe the
To do that, use the fact that the Periodic Table can be organized in blocks.
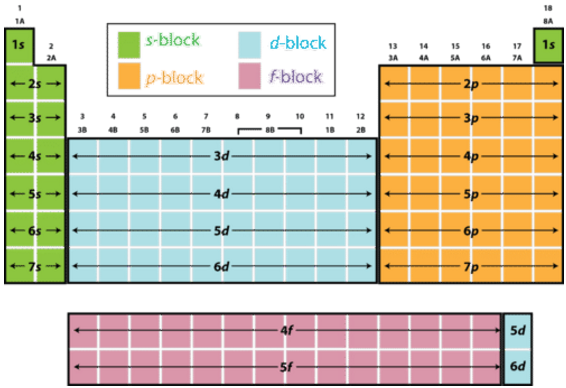
In your case, the last
This means that these electrons will have the angular momentum quantum number,
l = 1 -> describes an electron located in thep block
The period in which silicon is located will give you the value of the principal quantum number,
n = 3 -> describes an electron located on the third energy level
Now, in order to find the magnetic quantum number,
l = 1 implies m_l = { (-1 -> "the p"_xcolor(white)(.)"orbital"), (color(white)(+)0 -> "the p"_zcolor(white)(.)"orbital"), (+1 -> "the p"_ycolor(white)(.)"orbital") :}
In your case, the
m_l = -1 -> describes and electron located in ap_x orbital
Finally, the spin quantum number,
m_s = {( +1/2 -> "the electron is added to an empty orbital"), ( -1/2 -> "the electron is added to a half-filled orbital") :}
Since the
m_s = +1/2 -> describes and electron that has spin-up
Therefore, you can say that the quantum number set that describes the
n = 3, l= 1, m_l = -1, m_s = +1/2 This electron is located on the third energy level, in the
3p subshell, in the3p_x orbital, and has spin-up

