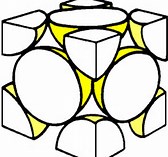
Given a face-centred unit cell as shown... Given a face-centred cubic cell as shown for an element, how many atoms does the unit cell contain?
1 Answer
Sep 17, 2017
Are there not 4 atoms per unit cell?
Explanation:
Each vertex of the cube shares
And so if the volume of a sphere is
And so occupied volume is
It has been a long time since I had to do this sort of stuff. But remember all I did was to consider the 8 vertices and 6 faces of the cube. Your inorganic text will tell you this definitively in the section on crystal packing and structure.

