Question #5a170
1 Answer
Ten electrons.
Explanation:
As you know, sulfur,
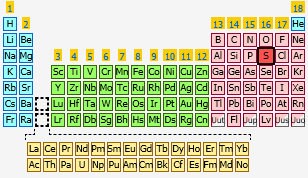
Now, the problem wants you to figure out how many electrons in a neutral sulfur atom can have
l = 1
For a sulfur atom, the principal quantum number,
n = {1, 2, 3}
The angular momentum quantum number,
l = {0, 1, ..., n - 1}
You know that
l = 1
designates the
 )
)
Each group located in the
For sulfur, you can have
Notice that for period
This means that on its second energy level, a sulfur atom has its
3 color(red)(cancel(color(black)("orbitals"))) * "2 e"^(-)/(1color(red)(cancel(color(black)("orbital")))) = "6 e"^(-)
Now, for period
The trick here is to realize that according to Hund's Rule, every orbital located in a subshell must be half-filled before any of the orbitals can be completely filled.
This means that for period
 )
)
This means that for period
overbrace(1 color(red)(cancel(color(black)("orbital"))) * "2 e"^(-)/(1color(red)(cancel(color(black)("orbital")))))^(color(blue)("completely filled")) + overbrace(2 color(red)(cancel(color(black)("orbitals"))) * "1 e"^(-)/(1color(red)(cancel(color(black)("orbital")))))^(color(blue)("half-filled")) = "4 e"^(-)
Therefore, the total number of electrons located in the
overbrace("6 e"^(-))^(color(blue)("in the 2p subshell")) + overbrace("4 e"^(-))^(color(blue)("in the 3p subshell")) = color(darkgreen)(ul(color(black)("10 e"^(-))))

