What hybridization is generally utilized by the central atom in a square planar molecule?
1 Answer
Always think about the directionality of each orbital when considering the hybridization. Their directionalities should add up vectorially to give you the appropriate directions and dimensionalities.
I got
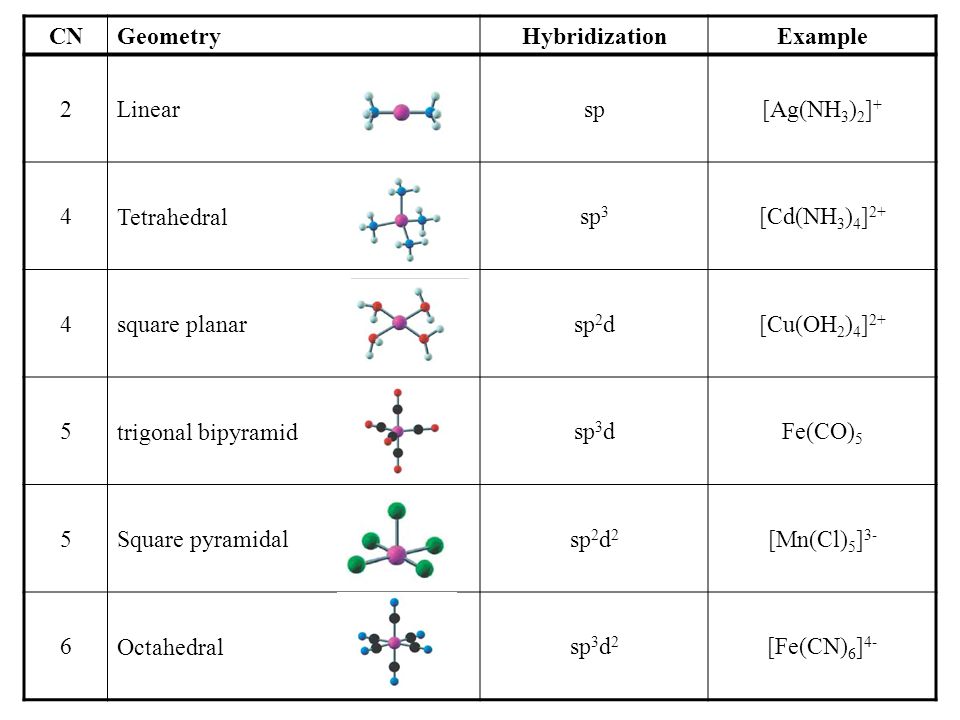
DETERMINING THE GEOMETRY
One thing to be careful about is what the
Since each
It is known that
DETERMINING THE HYBRIDIZATION
The hybridization should be based on the geometry. We let the complex be on the
- The
#6s# orbital is involved by default. Treat it as if it were an "identity" element. - Given that the
#6p_x# and#6p_y# orbitals also lie along the coordinate axes, they would also be involved. - Given that the
#5d_(x^2-y^2)# orbital lies along the coordinate axes (where the ligands lie as well), the#d_(x^2-y^2)# has to be involved.
Thus, the hybridization is

