Question #0205d
1 Answer
Oct 4, 2017
Explanation:
Atomic number of Zinc is 30.
So, according to octet rule
Maximum electrons in shell K
Maximum electrons in shell L
Maximum electrons in shell M
Number of electrons left for shell N, is
Therefore Zinc need to get rid of 2 electrons and it will become a 🐈ion.
So, the valency of Zinc cation is 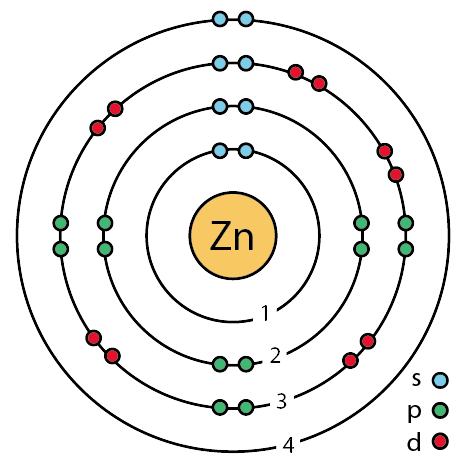 Wikimedia commons
Wikimedia commons

