What is the electron configuration of mercury?
1 Answer
Oct 29, 2017
Examine your periodic table and find mercury.
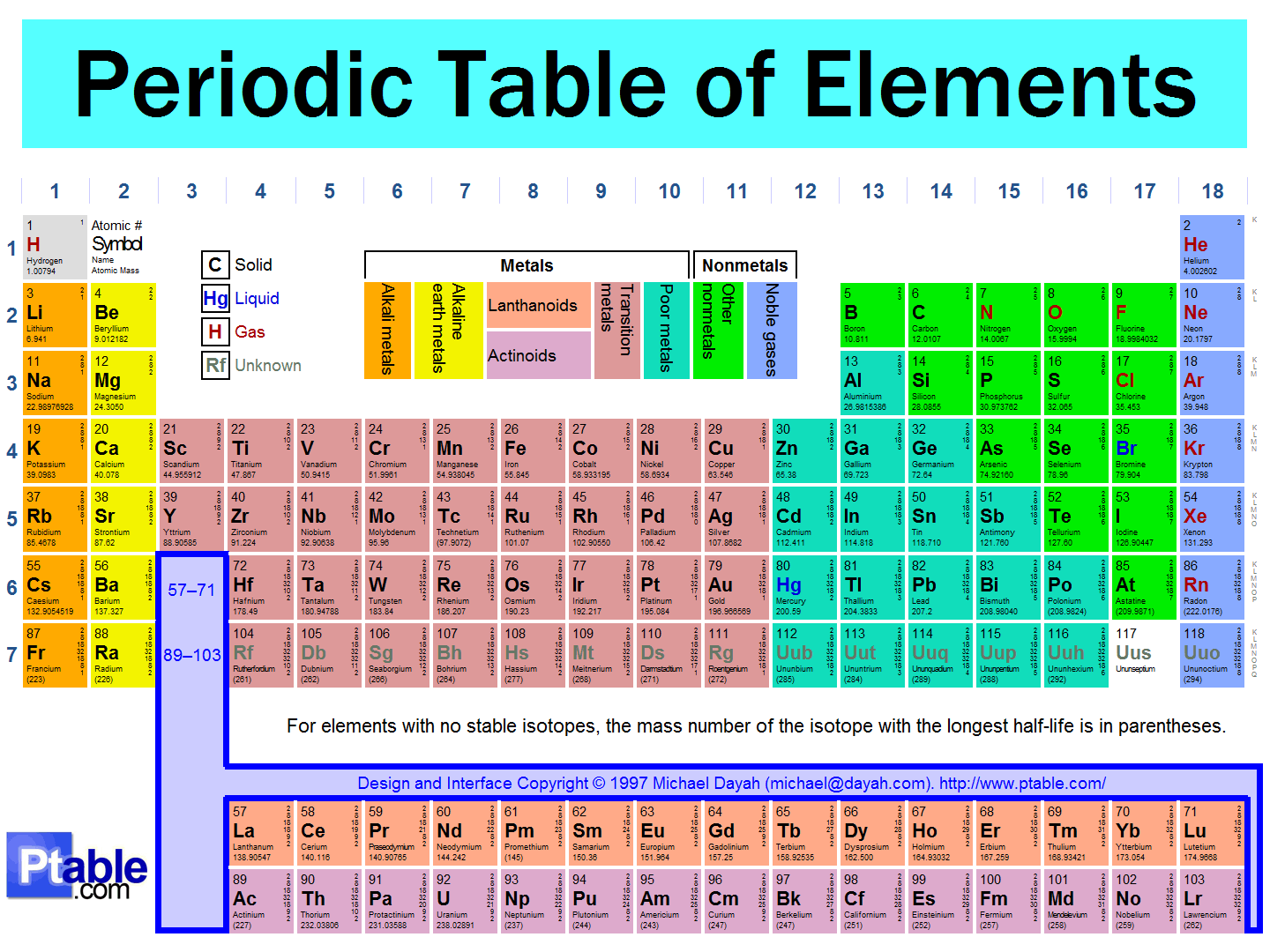
You should then find its atomic number is
For the 6th row of the periodic table, we introduce the
Mercury has a regular electron configuration. It becomes:
#color(blue)(["Xe"] 4f^14 5d^10 6s^2)#

