Question #86f10
1 Answer
There are 2: D-Glucose and L-Glucose..
Explanation:
Glucose is a hexose.
Hexoses have 6 Carbon atoms. Because 4 of the 6 carbon centres are Chiral , there are 16 possible configurations.
BUT: Not all of these are called Glucose.
Have a look at the Fischer projection of (D-)Glucose:
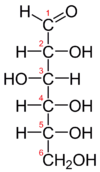
The central four C-atoms (2 to 5) are chiral, meaning you can swap the H- and OH-groups around. If you swap, lets say, the groups of C2 around, you will get:
Unfortunately, this is not Glucose, but Mannose...
Because tere are 4 Chiral C-atoms, there are 16 possible configurations. Half of these are the exact mirror image of each other, like a pair of gloves: identical, but not super-imposable.
That leaves us with 8 pairs of Enantiomers, and these are considered different sugars. They are: Altrose, Allose, Mannose, Galactose, Idose, Gulose, Talose and our Glucose.
As mentioned, each of these pairs exists as either a left-hand "glove", or a right hand glove identity.
This makes them optically active, and they are either called D- (Dextro-) or L- (Levo-) rotatory.
So, for Glucose: only two...

