Question #4d93a
1 Answer
Sometimes not being able to do something is because there is a tiny bit of understanding that is not correct which throws the whole thing off! Don't worry, just work back from basics and go from there.
Explanation:
A lewis structure (or lewis dot structure) is just a way of drawing a molecule with atoms electrons shown. It is a way of seeing how atoms electrons are interacting and the lone pairs available on certain atoms. It can help you decide how many bonds an atom should have and thus what elements it can bond with etc.
Lets take oxygen (
Oxygen is in group 6 in the periodic table. This means it has 6 valence electrons (or 3 electron 'pairs' in its outermost shell) This would be drawn as.
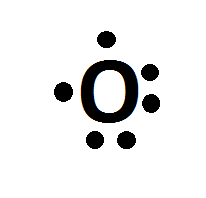
The atoms atomic symbol, in this case O. The dots each represent one electron. To fulfil the octet rule (i.e. be in a low energy state with 8 electrons in its outermost shell), Oxygen can make a bond with two atoms that have a valency of 1 (i.e. Hydrogen -

(Here, you can see that the hydrogen 'donates' 1 electron (red) to the oxygen atom to give themselves a stable pair and the oxygen atoms 'receive' these two electrons to give it 8 outer electrons.
Oxygen can also bond to an atom that has a valency of 2 (i.e Magnesium -
It can also, however 'share' another electron pair with an atom that also has a valency of 6 (i.e. Oxygen -
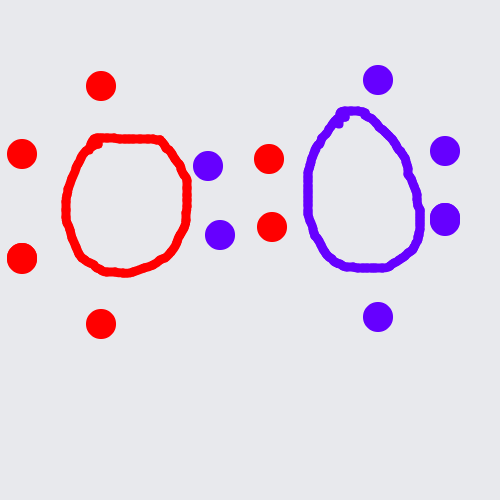
The key thing to be aware of when drawing lewis structures is the valency (how many electrons are in an atoms outermost shell) of each atom involved in the molecule being drawn. The valency can be seen easily by finding out which group (i.e. column) an atom is in within the periodic table.

