Question #b15f5
1 Answer
You're close!
Explanation:
The name you have written down for this compound is actually a little off because anionic ligands follow a different naming convention.
When an anion that ends in -ide, like the chloride anion, is a ligand, its suffix changes to -o. So instead of dichloride, you must say dichloro.
Also, keep in mind that the name of the ligands and the name of the metal cation are written without added spaces. So instead of
diammine dichloro platinum(II)
you should have
diamminedichloroplatinium(II)
And that's pretty much it.
#"Pt"(color(red)("NH"_ 3))_ color(green)(2) color(darkorange)("Cl")_ color(blue)(2) -> color(green)("di")color(red)("ammine")color(blue)("di")color(darkorange)("chloro")"platinum"("II")#
Notice that you're dealing with a neutral compound here because the
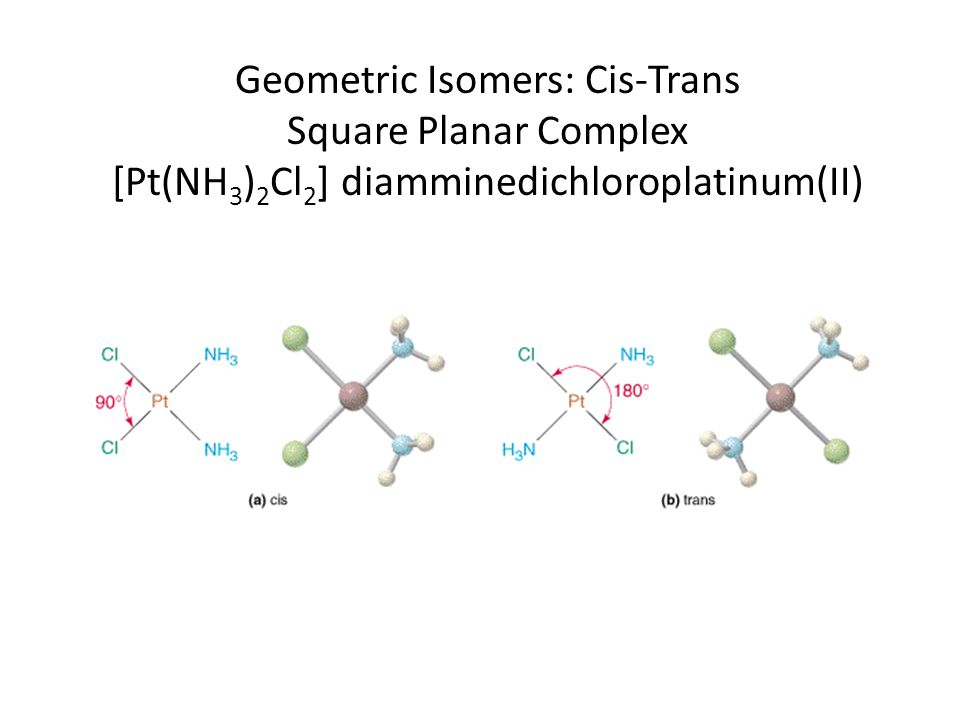
As a final note, it's worth pointing out that this compound has a cis isomer and a trans isomer and that its cis isomer is actually known as cisplatin