Question #496a7
1 Answer
Here's what I got.
Explanation:
For starters, the units you have for the change in entropy is incorrect. The change in entropy is usually measured in joules per Kelvin,
#DeltaS = - "80 kJ"#
is actually
#DeltaS = - 80 color(red)(cancel(color(black)("J"))) "K"^(-1) * "1 kJ"/(10^3color(red)(cancel(color(black)("J")))) = -"0.080 kJ K"^(-1)#
As you know, we can use the change in Gibbs energy,
#color(blue)(ul(color(black)(DeltaG = DeltaH - T * DeltaS)))#
Here
#DeltaH# is the change in enthalpy of the reaction#T# is the absolute temperature at which the reaction takes place#DeltaS# is the change in entropy of the reaction
Now, in order for a reaction to be spontaneous at a given temperature, you need to have
#DeltaG < 0#
In your case, the reaction is exothermic because its change in enthalpy is negative.
#DeltaH < 0 implies "exothermic reaction"#
The change in entropy is negative, which means that the disorder of the system is actually decreasing.
#DeltaS < 0 implies "decreasing disorder"#
This tells you that your reaction is enthalpy driven, which is what happens when an exothermic reaction overcomes a decrease in entropy.
In such cases, the spontaneity of the reaction depends on the temperature at which the reaction is taking place.
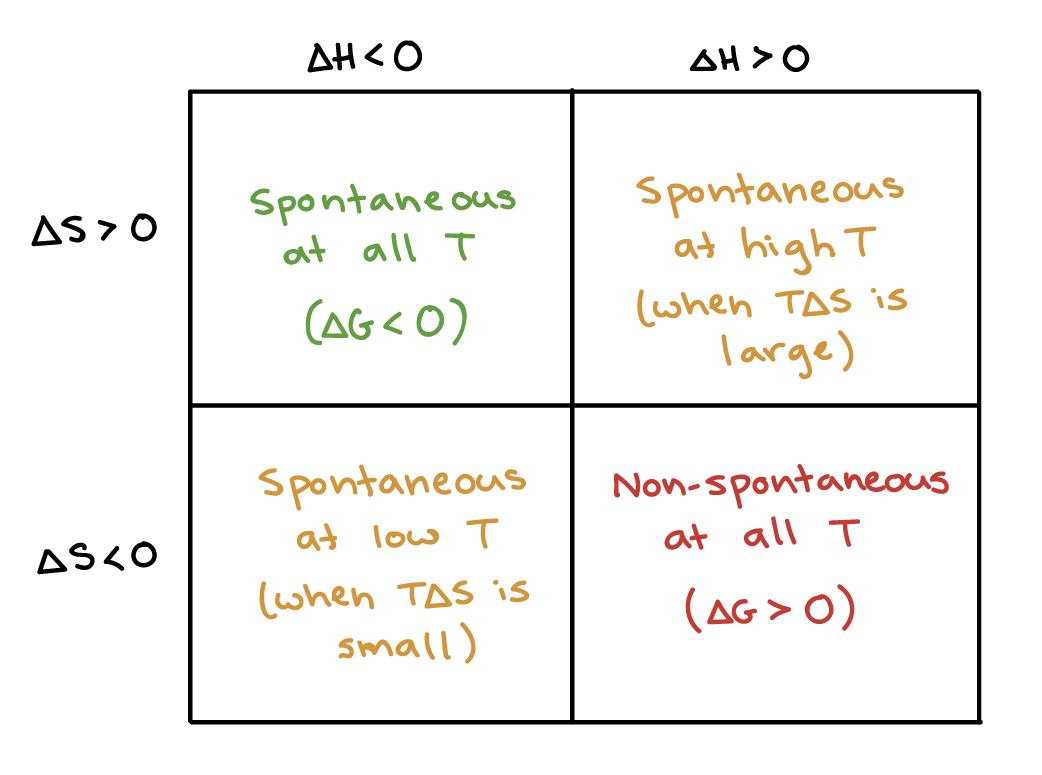
In your case, you have
#DeltaG = overbrace(DeltaH)^(color(blue)(<0)) - overbrace(T * DeltaS)^(color(blue)(<0))#
For
#DeltaG = DeltaH + T * |DeltaS|#
so in order for the reaction to be spontaneous, you need
#DeltaG < 0 implies T * |DeltaS| < |DeltaH|#
#T < (|DeltaH|)/(|DeltaS|)#
At equilibrium, you have
#DeltaG = 0 implies T * |DeltaS| = |DeltaH|#
which gets you
#T = (DeltaH)/(DeltaS)#
Plug in your values to find
#T = (-50color(red)(cancel(color(black)("kJ"))))/(-0.080color(red)(cancel(color(black)("kJ")))"K"^(-1)) = "625 K"#
This means that your reaction is
#"spontaneous " => " T < 626 K"# #"nonspontaneous " => " T > 625 K"# #"at equilibrium " => " T = 625 K"#
So you can say that when the temperature increases and reaches
#"625 K" = "625 K" - 273.15 = 352^@"C"#
your reaction goes from being spontaneous to being nonpontaneous.

