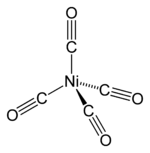
How would we know that "Ni"("CO")_4 prefers tetrahedral over square planar?
1 Answer
Both have all paired electrons. The only difference is their stability.
Here is its MO diagram (it is tetrahedral):
Here, the
The square planar splitting diagram (blank) would also be filled completely:
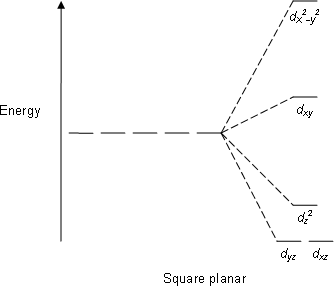
In comparing tetrahedral vs. square planar
In this case, from the basis of the angular overlap method alone, there is no preference between tetrahedral vs. square planar; the destabilization energy relative to the free-ion field is
However, since nickel is a small transition metal, we expect it to favor tetrahedral over square planar, and it does.
Since the
The tetrahedral structure has minimized the anticipated ligand-ligand bonding-pair repulsions, and that is seen in how small